Accelerated Antibody Discovery and Expression
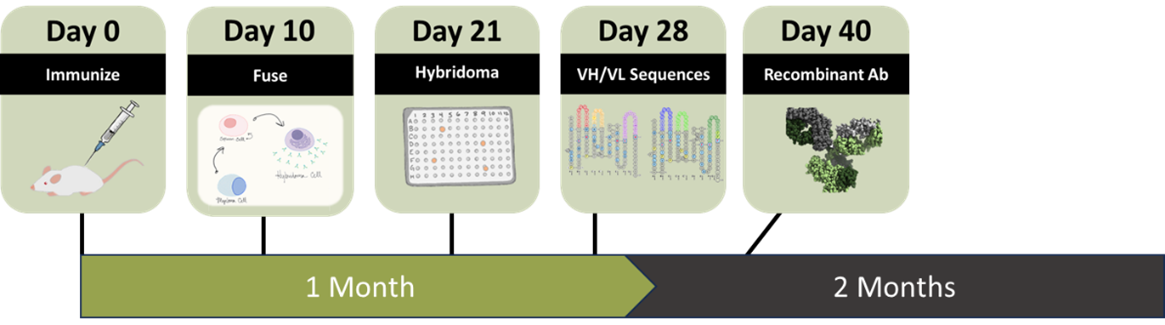
Immunization to Recombinant Antibodies in 40 days
Need antibodies fast? Our internally developed single immunization protocol (SIMM) generates hybridomas in 21 days, VH/VL sequence in <30 days, and recombinant antibody in as fast as 40 days.
SIMM + Accelerated Expression
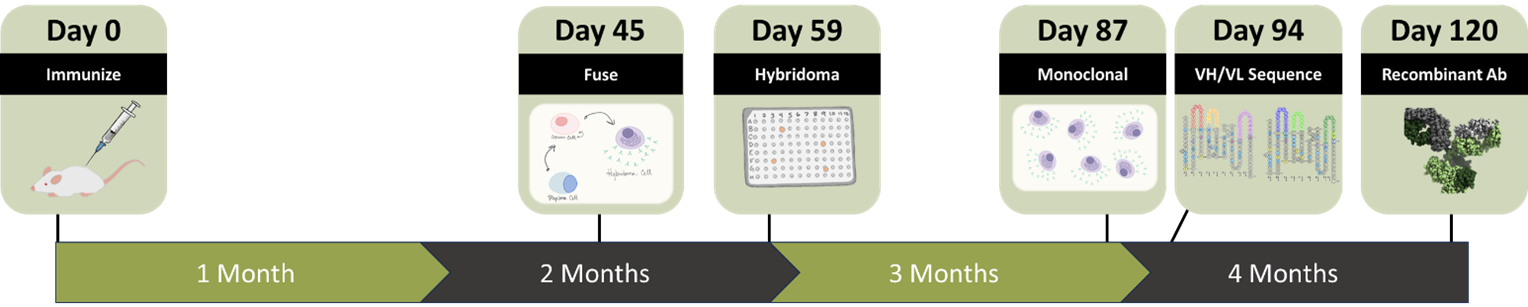
Compare to standard processes
Timeline to validated antibody is comparable to single cell cloning, while still getting the cost-conscious advantages of hybridoma. Furthermore, since antibody activity is first confirmed as a hybridoma, this approach boosts a high reproducibility rate of antigen binding (100% of parental wells cloned) compared to single cell cloning.
After less than 2 months, you can have monoclonal hybridomas, validated sequence, and recombinant antibodies to move forward.
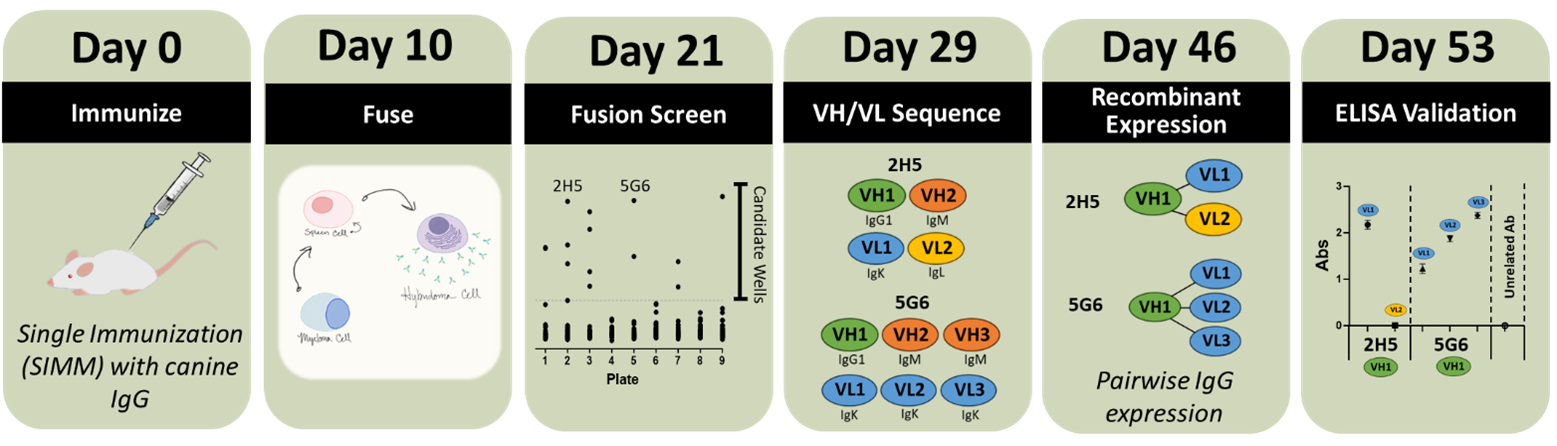
Case Study 1: Antibodies to Canine IgG in 53 days
Project Goal: A recombinant antibody to canine IgG as quickly as possible
We utilized a single immunization and generated hybridoma on Day 10. On Day 21, antibodies binding to canine IgG were identified through solid phase ELISA.
Candidate wells were expanded and rescreened for specificity to canine compared to human IgG. For accelerated expression, we sequence a mixed population and pairwise express all possible heavy and light chain combinations to identify the best antibodies.
Two candidate wells were sequenced using our rapid VH/VL sequencing, which confirmed a mixed population with two heavy chains in one well and three in the second. Since we screened only for IgG antibodies, the IgM were eliminated as possible binders. The remaining heavy chain sequences were expressed with each light chain.
Recombinant antibody derived from each parental well validated canine IgG binding. We compare the different heavy and light chain combinations for titer and affinity using biolayer interferometry and efficiency of purification to identify the best combination for the customer. After completing the characterization, the customer can be ready to move forward with large scale production in less than 2 months.
Case Study 2: Reagent Antibodies for Evaluation of Drug Levels and Activity in Patient Sera
Antibodies for Detection and Neutralization of Biotherapeutics
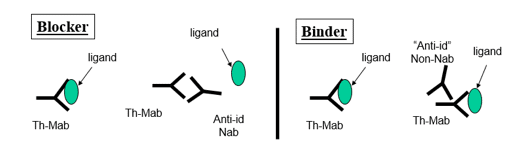
There is a need for antibodies in pre-clinical and clinical studies for the detection of potential biotherapeutic antibodies (Th-Mab)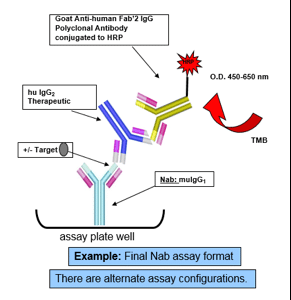 and also for the blocking of the therapeutic to its specific ligand target (anti-idiotypic/neutralizing (anti-ID Nab)).
and also for the blocking of the therapeutic to its specific ligand target (anti-idiotypic/neutralizing (anti-ID Nab)).
As there is a potential for adverse effects on the animal’s immune system from either the active drug-antigen and/or resulting circulating antibody during the immunization phase, specifically designed immunization protocols are used to abrogate potential adverse effects.
Case Study 3: Reagent Antibodies for Evaluation of Drug Levels in Patient Sera
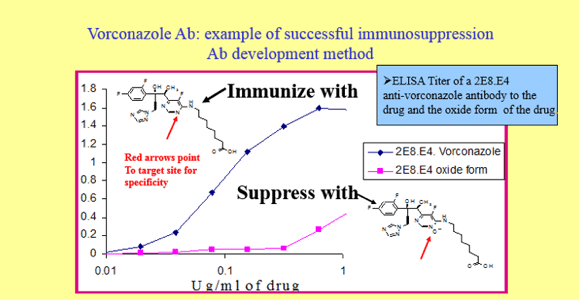
Antibodies Specific for Parent Small Molecule Drug Over Potential Metabolites
Mice were immunosuppressed with the metabolite (oxide form of the drug) and then challenged with the parent drug. Following fusion, clones producing specific antibody to the parent drug were selected over clones producing antibodies to the metabolite. An ELISA was then developed using the parent drug specific antibody to detect drug in patient sera samples.
Successful Green Mountain Antibodies Targets
- Small molecules (including drug candidates)
- Other small synthetic chemical entities (cGMP, cAMP, therapeutics, others)
- Biotherapeutics (Biosimilars)
- Proteins
- Peptides
- Ion channels/transporters
- GPCR’s
- Membrane targets
- Pre-clinical/Clinical
- Antibodies for PK/PD
- Antibodies for drug level measurements
- Antibodies for neutralization assays
- Antibodies for post marketing validation
- VLP’s, proteoliposomes, nanoparticles
- DNA/RNA
- Glycans/carbohydrates
- Drug delivery systems (CAR T, ADC, others)
Green Mountain Antibodies – Notable Successes
- Reagent antibodies for Pre-clinical and Clinical studies (ADA, PK, Tox, Neutralizing, functional evaluation)
- Small molecule therapeutic development studies (Zeljanz, Voriconazole, Azithromycin, Cortisol, cyclophosphamide)
- Functional antibodies (agonist, antagonist)
- Reagent antibodies for various assay platforms: immunohistochemistry, flow cytometry, Western analysis, immunoprecipitation, ELISA, MSD, Gyros, etc.
- Reagent antibodies for pharmacodynamics studies
- Reagent antibodies for evaluation of drug levels in sera (antibodies specific for parent drug over potential metabolites)
- Reagent antibodies that distinguish specific arms of bi-specific antibodies
- Antibodies for biotherapeutic candidate development
- Antibodies for diagnostic kit development
- Reagent antibodies for quality control evaluation of marketed drug products
- Internal product line development