Customized anti-idiotypic antibody services
GMAb offers monoclonal and polyclonal antibody development to support custom anti-idiotypic antibody campaigns. We approach monoclonal antibody development by immunizing mice or rats with the therapeutic molecule like a whole antibody, Fab, scFv, or VHH. and screening against the therapeutic and an appropriate irrelevant IgG or framework control. Antibodies with specificity to the therapeutic’s unique variable region, known as anti-idiotypic antibodies (anti-ID), are expanded. Neutralizing ability can be assessed by determining if the antibody can block the therapeutic molecule from binding its target ligand. Knowing the purpose and final assay goals will assist in identifying hybridoma screening strategies. Immunogenicity assays, including PD assays, PK assays, and ADA assays are common applications.
______________________________________________________________________________
Types of anti-idiotypic antibodies
Explore More Services
- Mouse Monoclonal Antibodies: Hybridoma Development
- Repetitive Immunizations Multiple Sites (RIMMS)
- ELISA Assay Development
- Gator Analysis

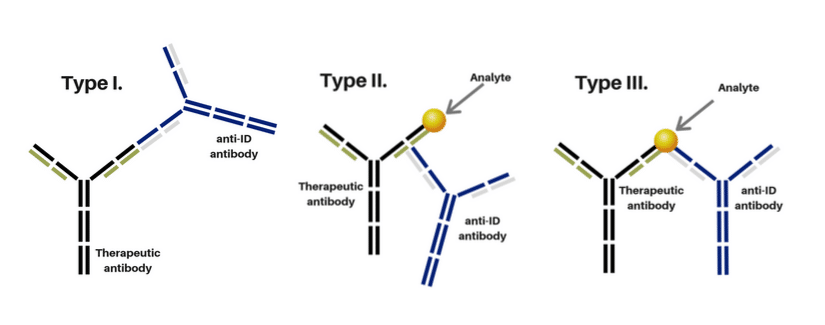
- TYPE I: Antibody detects free antibody drug. Antibodies used in a neutralizing assay (nAb Assay)
- TYPE II: Antibody detects bound, partially bound and free drug antibodies; not inhibitory.
- TYPE III: Antibody detects bound antibody drug only. Measure the antibody drug/target complex. Requires additional immunization campaign.
A similar strategy is employed for the development of anti-idiotypic polyclonal antibodies in either rabbits or goats. The animals are immunized with the therapeutic. Antisera is collected over the course of 6 months, with the first purification occurring during month 2. The antibody is affinity purified against the drug, followed by a depletion column against Hu IgG; yielding highly purified material against the target.
Both monoclonal and polyclonal projects can be run in tandem, providing a tool set of antibodies that can be used through pre-clinical and clinical trials.
______________________________________________________________________________
Support Through Pre-clinical & Clinical Trials
Over the last 15 years the introduction of immune therapies, which includes immuno-oncology, has greatly increased the need for custom anti-idiotypic antibody production. These therapies have changed the landscape of clinical trials and the drug pipeline. Over 500 monoclonal antibody targets have been investigated. The timeline from concept to clinic is now only one year. One out of 4 monoclonal therapies are receiving FDA approval, where one out of 8 small molecules receive the same result.
______________________________________________________________________________
Monoclonal Lead Generation and Discovery
In addition to anti-idiotypic projects, GMAb assists translational science groups in creating tool antibodies and monoclonals against biomarkers for discovery lead generation. Such projects require increased diversity, where maximizing the number of antibody candidates is very important. Our strategy is to cast a wide net and incorporate multiple strains of animals, targeted immunization protocols, such as RIMMS, and complex ELISA assay development.
