Rely on Green Mountain Antibodies for your recombinant antibody expression. We can take your project all the way from an idea to the ideal recombinant antibody for your application with our best-in-class hybridoma antibody discovery, variable domain sequencing, and recombinant expression services.
Transient antibody production in HEK293 cells is suitable for small scale production and antibody screening to move your project forward as quickly as possible, without the need and cost of developing a cell line.
Our process provides up to 5 mg of purified antibody, validated for antigen binding, to support your therapeutic antibody discovery program, starting at 3 weeks from sequence.
By constructing a recombinant antibody, you are no longer limited to the antibodies produced by the immune system. We can create the optimal format for your application including scFv, Fab, bispecific, different isotype or subtype, or switch species. We can incorporate changes to your sequence that will reduce immunogenicity (humanization) or modulate immune response through Fc sequence optimization.
Our Approach
Start with an idea and let us guide you from antigen to antibody. Or bring us your hybridoma, B cell sequence, or plasmid and let us produce the recombinant antibody that you need.
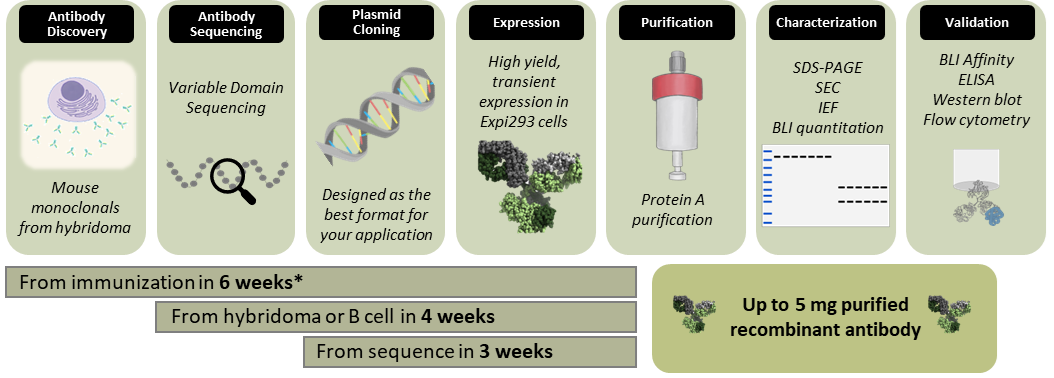
*Need antibodies fast? Go from immunization to validated recombinant antibody in as fast as 40 days using our Accelerated Antibody Discovery and Expression services.
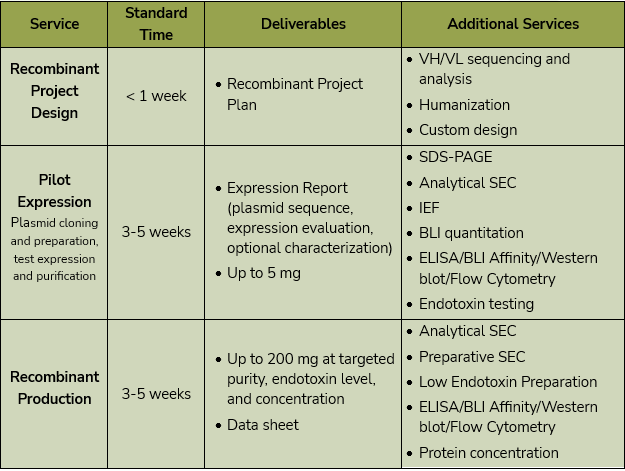
From this initial pilot, we can analyze production, purity, and aggregation using biolayer interferometry (BLI) quantitation, size exclusion chromatography (SEC), and SDS-PAGE. We can perform isoelectric focusing (IEF) to determine the ideal buffer conditions for your purified antibody. We provide samples of the antibody to test in your own assays, or we can assay them here through ELISA, BLI affinity, western blot, or flow cytometry to compare different sequences and ensure that your antibody will work for your application.
From this detailed evaluation and characterization, we can scale up your validated antibody to produce up to 200 mg at the concentration, purity, and endotoxin level needed. We can perform preparative SEC to ensure the final product meets your specifications.
Why Green Mountain Antibodies?
Integration: From idea to characterization and validation. We can discover the antibody, sequence your variable domains, express the antibody recombinantly, and assay for your specific application to produce the optimal antibody.
We know more: We have over 25 years’ experience in the production and purification of monoclonal antibodies and can apply our scientific knowledge, optimized protocols, and skilled workforce to our recombinant expression. An experienced scientist will be involved in your project from conceptualization to delivery, able to work with you to craft the plan needed to develop and validate that antibody that you need.
Speed: Biotechnology is a fast-paced field, and your time is important. By integrating with our antibody sequencing and assays workflows, we can get your recombinant antibody faster. Further, using our internally developed single immunization protocol, we can accelerate your project from immunization to recombinant antibody in 6 weeks.
Formats: You are not limited to the antibodies produced by the immune system by using our recombinant expression services. We can create the optimal format for your application including scFv, Fab, or bispecific. We can switch isotypes, subtypes, or species to fit your needs and allow you to test the ideal format. We can incorporate changes to your sequence that will reduce immunogenicity, such as humanization for potential therapeutics, or modulate immune response through Fc sequence optimization.
Service Details
Advantages of Recombinant Antibodies
- Known sequence during screening
- Properties are due to the specific sequence with no additional heavy or light chains to complicate analysis
- Batch-to-batch consistency: more reproducible results
- No cell-line drift and mutations
- Rescue monoclonal antibodies from unstable cell lines
- Modify existing antibodies
- Troubleshoot specific properties such as aggregation or enhance binding
- Design for a specific purpose – create exactly what you need for an assay or therapeutic: switch species, change isotype, reduce immunogenicity
- Develop new antibodies
- antigens that are toxic or non-immunogenic in animals
- directly from B cell or AI sequence
Additional Services