GMA-340
Murine Anti- GPIba
Clone GMA-340
Background
Platelet membrane glycoprotein Ib (GPIba) is comprised of an alpha and beta subunit linked by disulfide bonds. GPIba (also known as CD42b) is a 135 kDa membrane protein subunit that binds a variety of adhesive and procoagulant ligands, including von Willebrand factor. Cleavage of GPIba by the “sheddase” ADAM17 releases the ectodomain glycocalicin into plasma. ADAM17 cleaves GPIba at Gly464-Val465. Liang et al. have shown that the murine monoclonal antibody designated 5G6 (GMA-340) binds the ADAM17 cleavage site and blocks glycocalicin release.
More Information:
Product Datasheet, pdfDescription
Species Reactivity: Human
Source: Murine
Specificity: Shedding cleavage site of GPIba
Immunogen: Human GPIba peptide (Ac-ELDQPPKLRG VLQGHLESSRNDPFC- amide) conjugated to ovalbumin.
Isotype: IgG1
Application
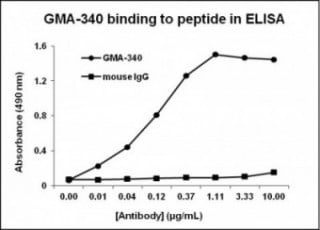
ELISA: Binds immobilized human platelet GPIba and synthetic peptide
Immunoblotting: Binds under reduced and non-reduced conditions
Data:
Purchase GMA-340
Antibody References:
- X. Liang, S.R. Russell, S. Estelle, L.H. Jones, S. Cho, M.L. Kahn, M.C. Berndt, S.T. Bunting, J. Ware and R. Li. Specific Inhibition of Ectodomain Shedding of Glycoprotein Iba by Targeting its Juxtamembrane Shedding Cleavage Site (2013) J Thromb Haemost. 11(12): 2155–2162.
- R.W. Colman. Major platelet Glycoproteins: Platelet Glycoprotein Ib-IX-V (2006). Hemostasis and Thrombosis: Basic Principles and Clinical Practice. Philadelphia, PA: Lippincott Williams & Wilkins.
- R. Li and, J. Emsley. The Organizing Principle of Platelet Glycoprotein Ib-IX-V Complex (2013) J Thromb Haemost. 11(4): 605–614.
- E.E. Gardiner, D. Karunakaran, Y. Shen, J.F. Arthur, R.K. Andrews And M.C. Berndt. Controlled Shedding of Platelet Glycoprotein(GP)VI and GPIb-IX-V by ADAM Family Metalloproteinases. (2007) J Thromb Haemost; 5: 1530–7.
