GMA-012
Murine Anti- Factor VIII
Clone GMA-012
Background
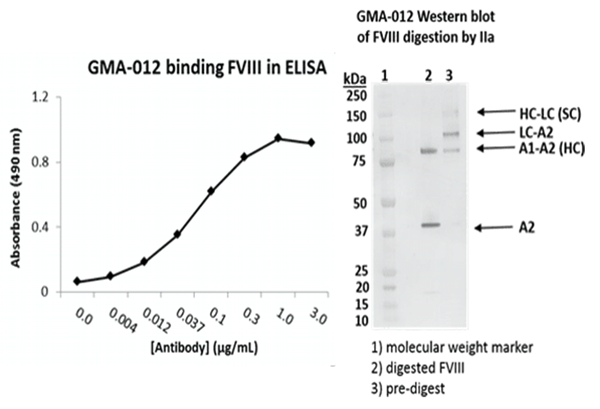
Factor VIII (FVIII) is a heterodimer consisting of a heavy chain (ranging in mass from 90 to 200 kDa) bound via metal ions to a light chain (80 kDa). In plasma, factor VIII circulates in an inactive form bound to von Willebrand factor. Following activation by factor Xa or thrombin, factor VIIIa can function as cofactor for the enzyme factor IXa in the activation of factor X in the presence of phospholipid and Ca2+. Absent or defective FVIII is the cause of the X-linked recessive bleeding disorder hemophilia A. GMA-012 (also known as R8B12) recognizes the discontinuous epitope of residues 497-510 and 584-593 in the A2 domain of FVIII,1 and is suitable for purification of FVIII,2 ELISA, sandwich ELISA3, Western blotting, and bio-layer interferometry applications.
More Information:
Product Datasheet, pdfDescription
Application
Data:
Purchase GMA-012
Antibody References:
- C. Ansong et al. (2006). J Thromb Haemost. 4:842–847.
- P. Lollar et al. Factor VIII and factor VIIIa. (1993). Method Enzymol. 222:128-143.
- H. Wakabayashi et al. (2010). J Biol Chem. 285(33):25176-25184.
- P. Fay et al. (1991). J Biol Chem. 266:8957-8962.
- R.C. Markovitz et al. (2013). Blood. 121(14):2785-2795.
- C.D. Scallan. (2003). Blood. 102(12):3919-3926.
- J.L. Plantier et al. (2010). J Thromb Haemost. 8 (2):286-293.
- L. Chen et al. (2009). Mol Ther. 17(3):417-24.
- J.L. Newell et al. (2009). J Biol Chem. 284:11080-11089.
- J. Yong et al. (2019). eLife. 8(e49682):1-25.
- I. Biswas et al. (2018). Arterioscler Thromb Vasc Biol. 38(8):1868–1877.
- M Monaghan et al. (2016). J Thromb Haemost. 14(5):1021–1030.
- L. Leong et al. (2015). Blood. 125(2):392–398.
- H. Wakabayashi et al. (2014). Thromb Haemost. 112(1):43–52.
- J. DeAngelis et al. (2012). The Journal of Biological Chemistry. 287(19):15409 –15417.
- J. DeAngelis et al. (2011). Thromb Res. 128(5):470–476.
- B. Mei et al. (2010). Blood. 116(2):270–279.
- S. Buddai et al. (2010). The Journal of Biological Chemistry. 285(8):5212–5223.
- J. Newell and P. Fay. (2008). Biochemistry. 47(33):8786–8795.
- J. Newell and P. Fay. (2007). The Journal of Biological Chemistry. 282(35):25367–25375.
- H. Wakabayashi et al. (2005). Biochemistry. 44(30):10298–10304.
- K. Nogami et al. (2005). The Journal of Biological Chemistry. 280(18):18476 –18487.
- K. Nogami et al. (2004). The Journal of Biological Chemistry. 279(32):33104–33113.
- K. Nogami et al. (2004). The Journal of Biological Chemistry. 279(16):15763–15771.
- H. Wakabayashi et al. (2004). The Journal of Biological Chemistry. 279(13):12677–12684.
- C. Manithody. (2003). Blood. 101(12):4802-7.
- M. Elnaggar et al. (2020). Molecular Therapy: Methods & Clinical Development. 17:1-12.
- H. Chun et al. (2020). J Thromb Haemost. 19(4):954-966.
